The US FDA authorised the emergency use of a new saliva based laboratory diagnostic test for coronavirus.
A key difference is the speed of the test, its being described as a 'game changer'.
The sped allows more people to access rapid easily.
Stephen Hahn, the Food and Drugs Administration Commissioner:
- "Providing this type of flexibility for processing saliva samples to test for COVID-19 infection is groundbreaking in terms of efficiency and avoiding shortages of crucial test components like reagents"
Its called SalivaDirect
- has been tested amongst US National Basketball Association (NBA) players and staff
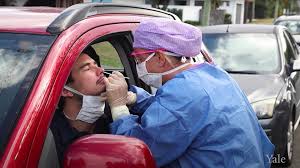
- simpler, less expensive, less invasive than nasopharyngeal (NP) swabbing
- yields similar outcomes as NP swabbing
Current testing:
---
ps. For clarity, this is a test for the infection, not a treatment. That is clear above, but wanted to restate it.



